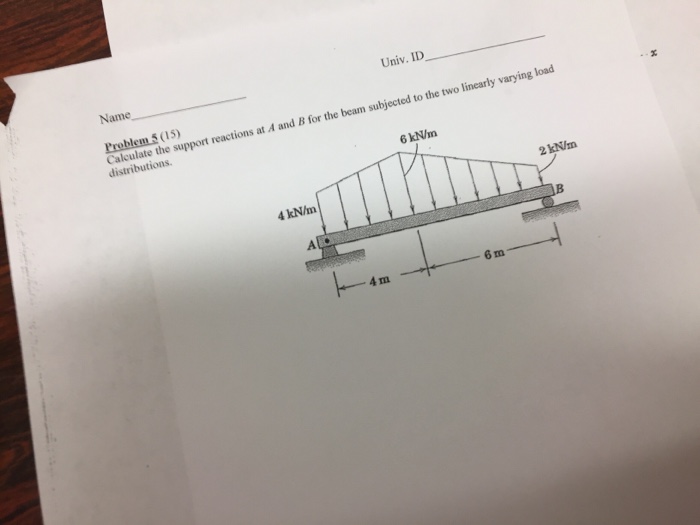
Interactive Session: OrganizationsCan Instacart Deliver?
The online grocery store Webvan was perhaps the most well-known flop of the dot-com boom. Its 2001 failure led many pundits and investors to conclude that the online grocery business model was untenable.
However, Webvan’s downfall was due mainly to pursuing a first-mover advantage strategy. It paid more than $1 billion to build huge distribution warehouses, bought fleets of delivery trucks, and invested heavily in marketing. Then it offered free deliveries on any size order, at virtually any hour, at prices that trumped its brick-and-mortar competitors. This was not a formula for generating profits.
In recent years other companies are testing the waters again for online grocery sales. FreshDirect in New York City has succeeded by combining fresh local produce, organic and kosher items, and custom-prepared meals with standard grocery store fare. Established brick-and-mortar firms including Albertson’s, Safeway and Peapod.com (the online entity for both Stop & Shop and Giant) took over as pure play online firms perished.
The newest entrant, Instacart bypasses the expenses of warehousing and transportation altogether by using a legion of independent contractors and local food retailers. These personal shoppers receive orders via the Instacart smartphone app, fill them from grocery store aisles, and use their own vehicles to deliver them to customers’ doors. Like fellow “sharing economy” firm Uber, Instacart minimizes labor costs by requiring its personal shoppers to pay for their own auto and health insurance and Social Security contributions. Purportedly paid between $15 and $20 an hour, depending on how quickly they can fill and deliver an order, most Instacart shoppers work part-time on flexible schedules.
Instacart co-founder and CEO Apoorva Mehta believes Instacart’s competitive advantage is twofold. First, customers are not limited to a single vendor and can combine items from multiple stores on one order, so product selection is truly customized. (Instacart uses special software that can track inventory across multiple supermarkets.) And since personal shoppers are on call around the clock, customers have to neither order many hours in advance of delivery nor wait for a delivery window. In fact, customers can have their grocery list filled and delivered in less than an hour!
Instacart’s app provides a detailed map of each local establishment including store aisle contents. The customer’s grocery list, compiled using extensive drop-down menus either on the website or in the app, is organized by merchant and aisle to provide maximum order fulfillment efficiency. Inventory is tracked for all of Instacart-affiliated merchants. As a personal shopper skims an aisle, bedecked in a bright green T-shirt flaunting the Instacart logo, items can be selected for different orders placed at different times. The software can also plan delivery routes and predict future customer orders.
iPhone users can connect to the Instacart app from Yummly, the largest recipe search engine in the world, and have the ingredients delivered in time for dinner. Visitors to Food Network websites, with more than half a million recipes, can browse recipes online and then click a button to add ingredients they need to their Instacart shopping cart. The Instacart app is integrated with Google Now cards so that Android users can place orders for either delivery or pickup using a token generated within the app.
Instacart’s core competencies thus dictate its target market: the price-insensitive, convenience shopper. At first, item prices were marked up (20 percent in one sampling) and a $3.99 delivery fee charged. An Amazon Prime–like service called Instacart Express requires a certain volume of business and a $99 yearly fee in exchange for free delivery. One of Webvan’s big mistakes was pursuing a mass-market strategy. It was never going to be able to turn a profit by providing quality and selection at rock-bottom prices—with free delivery to boot. Instacart is instead catering to shoppers who are willing to pay a premium to have both quality and selection.
By mid-2015 Instacart had 200 employees and 4,000 personal shoppers in New York, Los Angeles, San Francisco, San Jose, Washington, DC, Chicago, Boston, Austin, Seattle, Philadelphia, Atlanta, Boulder, Denver, Houston, and Portland, Oregon. It continues to grow. Grocery purveyors, from large chains such as Costco, BJ’s Wholesale Club, Safeway, Kroger, Super Fresh, Trader Joe’s, and Whole Foods to local specialty shops such as Erewhon Organic Grocer & Café in LA, Marczyk Fine Foods in Denver, and Green Zebra in Portland are now welcoming Instacart as a way to expand their customer bases ahead of the full national rollout of Amazon subsidiary Amazon Fresh.
While many analysts predict that matching the bargain basement prices of Amazon and Walmart is unavoidable, Instacart is instead modifying its business model. Partnerships with Petco and Tomlinson’s Pet Supplies in Austin, Texas, hint of additional product areas on the horizon, while Mehta speculates that expansion into general logistics is conceivable.
Many of Instacart’s grocery store partners now set their own prices, paying Instacart a cut of each order. This has freed Instacart of the burden of markups, protected it from the vagaries of variable food prices, and provided a more stable profit structure. Retailers have been willing to pay Instacart in the hope of gaining more business because Instacart enables a single store to serve people across a larger geographic area. Affiliated retailers are reporting gains, although the numbers are small. Nilam Ganenthiran, head of Business Development and Strategy, maintains that different types of agreements have been reached, declining to specify whether partners are outsourcing their e-commerce to Instacart for a monthly fee or are charged per item purchased, per order placed, or per customer serviced.
With national chains achieving just 1 to 2 percent margins on grocery delivery, the Instacart model of layering labor on top of the existing grocery infrastructure is still unproven. According to a Wall Street Journalanalysis, an order of 15 common items such as frozen peas, milk, cereal, and fresh fruit costing about $68 from a San Francisco Safeway store would produce a profit of only $1.50 for Instacart. If the order were smaller by one 28- ounce jar of peanut butter, Instacart would break even, and a smaller order could push it into the red. Without price concessions from participating merchants, can Instacart attract enough customers? And maintain a pay scale that ensures the topnotch customer service demanded by its target market? And still make a profit? And can retailers’ sales gains from Instacart be sustained? Instacart may be a great idea, but it’s a very big bet.
Case Study Questions
Analyze Instacart using the value chain and competitive forces models. What competitive forces does the company have to deal with? What is its value proposition?
Explain how Instacart’s business model works. How does the company generate revenue?
What is the role of information technology in Instacart’s business model?
Is Instacart’s model for selling online groceries viable? Why or why not?