Report Form Experiment 12 Titration of Vinegar Name: Chandell China Section: 122 Date: Nod 18th, ...
please help with calculations for all
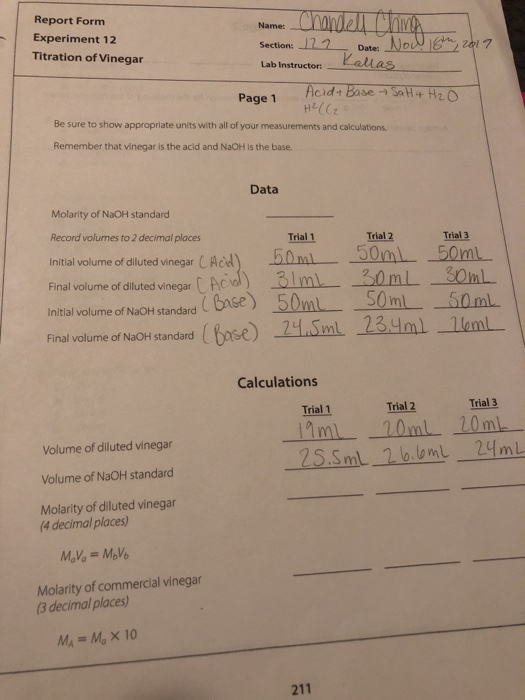

0.25 is the molarity of NaOH
Report Form Experiment 12 Titration of Vinegar Name: Chandell China Section: 122 Date: Nod 18th, 2017 Lab Instructor: Kallas Sa H4 H2O Acid+ Base H2((2 Be sure to show appropriate units with all of your measurements and calculations Remember that vinegar is the acid and NaOH is the base Data Molarity of NaOH standard Record volumes to 2 decimal places Trial 1 Trial 2 Trial 3 Initial volume of diluted vinegar CAcid) 50ml 30m 50ml Final volume of diluted vinegar Acid) 3 lm 30ml Soml andard (Base) 50mL 50mL 50mL Final volume of NaOH standard Base) 24.5ml 23.4ml 16ml Calculations Trial 1 Trial 2 Trial 3 19 ml - 20mL 20mL 25.5mL 26.6m 24mL Volume of diluted vinegar Volume of NaOH standard Molarity of diluted vinegar (4 decimal places) MoVo = Movo Molarity of commercial vinegar (3 decimal places) MA = M, X 10 2017
Page 2 Percentage of acetic acid in vinegar (2 decimal places) MAX M. x 600 x 100 100 See the pre-lab Information for the meaning of the numeric values. Average percent acetic acid concentration in vinegar (2 decimal places)
Solved
Chemistry
1 Answer
Mustafa Kent

Login to view answer.