Titration for Acetic Acid in Vinegar-Lab Report Exercise 1: Determining the Concentration of Acet...
Are my answers correct?
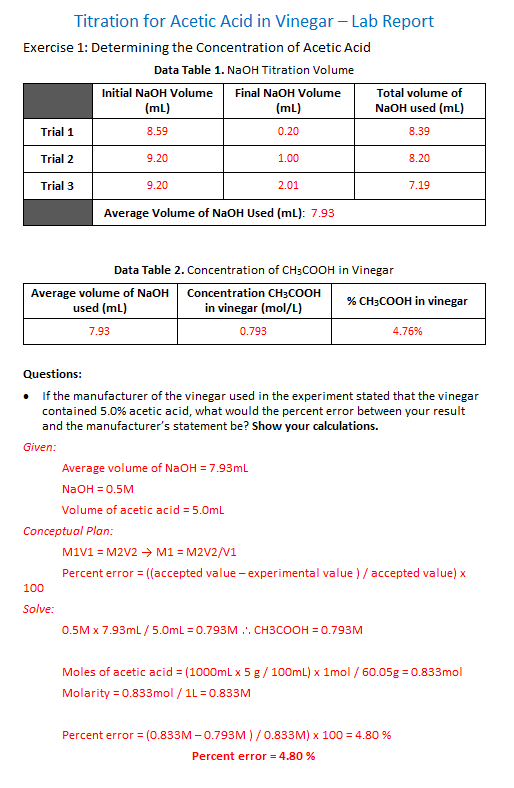

Titration for Acetic Acid in Vinegar-Lab Report Exercise 1: Determining the Concentration of Acetic Acid Data Table 1. NaOH Titration Volume Initial NaOH Volume (mL) 8.59 9.20 9.20 Final NaOH Volume Trial 1 Trial 2 Trial 3 (mL) 0.20 1.00 2.01 Total volume of NaOH used (mL) 8.39 8.20 7.19 Average Volume of NaOH Used (mL): 7.93 Data Table 2. Concentration of CH3COOH in Vinegar Average volume of NaOH Concentration CH3COOH % CH3C00H in vinegar used (mL) in vinegar (mol/L) 7.93 0.793 4.76% If the manufacturer of the vinegar used in the experiment stated that the vinegar contained 5.0% acetic acid, what would the percent error between your result and the manufacturer's statement be? Show your calculations. . Given Average volume of NaOH7.93mL Volume of acetic acid 5.0mL Conceptual Plan M1V1 M2V2 M1 M2V2/V1 Percent error((accepted value - experimental value)/accepted value) x 100 Solve 0.5M x 7.93mL 5.0mL 0.793M. . CH3COOH 0.793M Moles of acetic acid (1000mLx5 g/100mL) x 1mol /60.05g 0.833mol Molarity 0.833mol1L 0.833M Percent error-(0.833 M-0.793 M ) / 0.833 M) x 100-4.80 % Percent error = 4.80 %
What challenges would you encounter with the titration if you had used apple cider vinegar or balsamic vinegar as the analyte instead of distilled white vinegar? Explain your answer . Due to balsamic vinegar's dark tones, this experiment would be difficult to conduct. The color change would be almost impossible to spot. Diluting the vinegar solution with water would be a benefit, however, if water could not be added, a chemical indicator would have to be used Additionally, white vinegar is a pure vinegar substance, consisting of only acetic acid and water. Balsamic and apple cider vinegar, on the other hand, contain chemicals that would alter the results. How would your results have differed if the tip of the titrator was not filled with NaOH before the initial volume reading was recorded? Explain your answer . If the tip of the titrator was not filled with NaOH, the recording would be inaccurate. Instead of NaOH filling the pipet entirely, air bubbles would be falsely assumed as NaOH. The first 0.5 mL- 1 mL volume would be air How would your results have differed if you had over-titrated (added drops of NaOH to the analyte beyond the stoichiometric equivalence point)? Explain your . An over-titrated solution would lead to inaccurate results due to a high volume of NaOH. There would be an excess volume of NaOH needed to neutralize vinegar's acetic acid If a 7.0 mL sample of vinegar was titrated to the stoichiometric equivalence . point with 7.5 mL of 1.5M NaOH, what is the mass percent of CH3COOH in the vinegar sample? Show your calculations. Average volume of NaOH used = 7.93mL Moles of NaOH used = 1.5M x 7.93mL= 11.9mmol Mass of acetic acid in vinegar 11.9mmol x 60.0g/mol/1000 % CH3COOH in vinegar-0.80g x 100/5mL-16% CH3COOH 0.71g Why is it important to do multiple trials of a titration, instead of only one trial? Performing multiple trails of titration assists in reduce random error. There . could issue such as weighing error, incorrect readings, or false calculations. The most accurate answers come from averaging multiple attempts. A student did not read the directions to the experiment properly and mixed up where to place the NaOH solution and the vinegar. He put the vinegar in the titrator and the measured amount of NaOH in the beaker. He then added a drop of the phenolphthalein to the solution in the beaker . in ven a r res The solution is then reversed, going from pink to colorless. This experiment would give it's conductor wrong calculations.
Solved
Chemistry
1 Answer
hamza syed

Login to view answer.